Overview
Limulus amebocyte lysate (LAL) is intended for the detection of gram-negative bacterial endotoxins.
Details
| REF | WATLA |
|---|---|
| Composition | 87% Polivinyl, 6,5% PES, 6,5% CO |
| Width | 140 |
| Height | 100 |
Intended Use: Limulus amebocyte lysate (LAL) is intended for the detection of gram-negative bacterial endotoxins.
PYROSTAR™ ES-F is intended for the qualitative detection of endotoxins by gel-clot or quantitative detection by kinetic
turbidimetric methods.
Turbidimetric Technique Endotoxin Specific FDA approved
● Endotoxin-specific lysate, avoids false positive results from glucans
● Available in multi-tests vials or single-test vials
● Can be used as either a gel-clot or Kinetic-Turbidimetric Assay (KTA) reagent
● (KTA) assays can be performed in tube reader or microplate reader
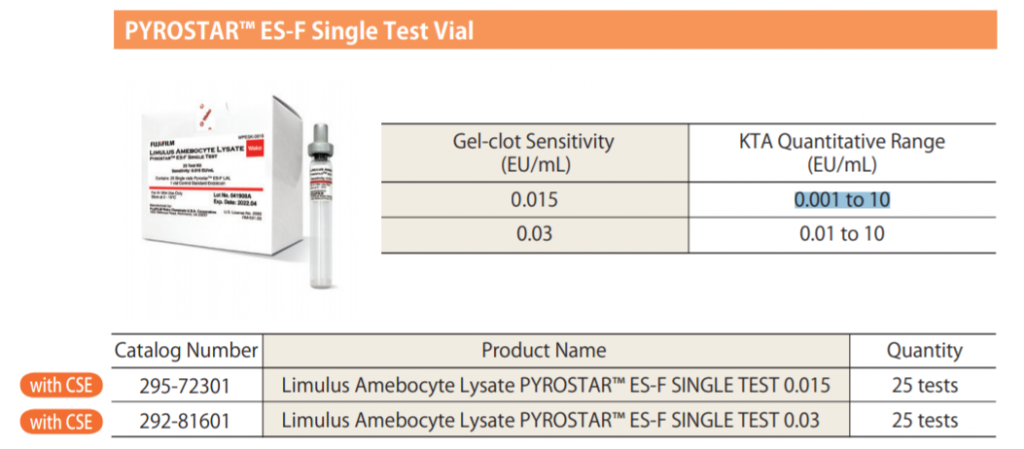
● Gel-Clot lysate sensitivities range from 0.015 to 0.25 EU/mL
● Available with matched control standard endotoxin (CSE)
● PYROSTAR™ ES-F reagents are available with a KTA quantitative range of either 0.001 EU/mL to 10 EU/mL.
The KTA quantitative range is related to the Gel-Clot sensitivity.
● 100uL sample size when used with tube reader; 50uL sample size when used with microplate reader
Limulus amebocyte lysate (LAL) is intended for the detection of gram-negative bacterial endotoxins.
| REF | WATLA |
|---|---|
| Composition | 87% Polivinyl, 6,5% PES, 6,5% CO |
| Width | 140 |
| Height | 100 |
Reviews
There are no reviews yet.